Animal cloning
What it is:
Making a genetic copy of an organism
In 1996, scientists announced the birth of Dolly the sheep. Dolly was the first mammal to be born that was an identical genetic copy of an adult organism – she was a clone. The news made headlines worldwide as people argued we were entering an age of science fiction with ethical implications that were both profound and far reaching. Many people feared the eventual cloning of humans, and many countries moved to ban the practice. Now, more than two decades later, cloning has not led to the clone war armies of Star Wars, and has largely moved out of the news headlines, but that doesn’t mean it is going away.
In biological terms, a clone is anything that is an identical genetic copy. Technically, identical twins are clones. Cloning many plants is as easy as taking a cutting from a branch or stem and getting it to grow roots. Any apple you have ever eaten came from a plant that did not grow from seed but was cloned in this simple way. Many organisms that reproduce asexually do so by making clones of themselves. Making a clone of an adult mammal, though, is much, much more difficult. Because most mammalian cells go through a process of development that generally can’t be reversed, the only cells that are capable of growing into a new whole organism are female egg cells. The trick in cloning is to somehow get a full set of adult DNA into a viable egg, and then to get that egg to start developing.
How it works:
Moving adult DNA to a reproductive cell
To create a clone of a mammal, a scientist starts with two cells: an adult cell containing the DNA from the animal to be cloned and an egg cell that will grow into the newly cloned offspring. This egg cell typically comes from an unrelated animal of the same species. Using a tiny pipette, the scientist pokes into the egg and sucks out the nucleus of the cell. The scientist then takes the nucleus of an adult somatic cell (a non-reproductive cell from the body) and injects it into the cytoplasm of the egg cell. The cells are then treated with specific chemicals or small electrical shocks to activate growth and cell division. After several rounds of cell division, the embryo will form a blastocyst, a ball-like structure of cells, that can be implanted into a surrogate mother. There the embryo will continue to develop just like any other fetus.
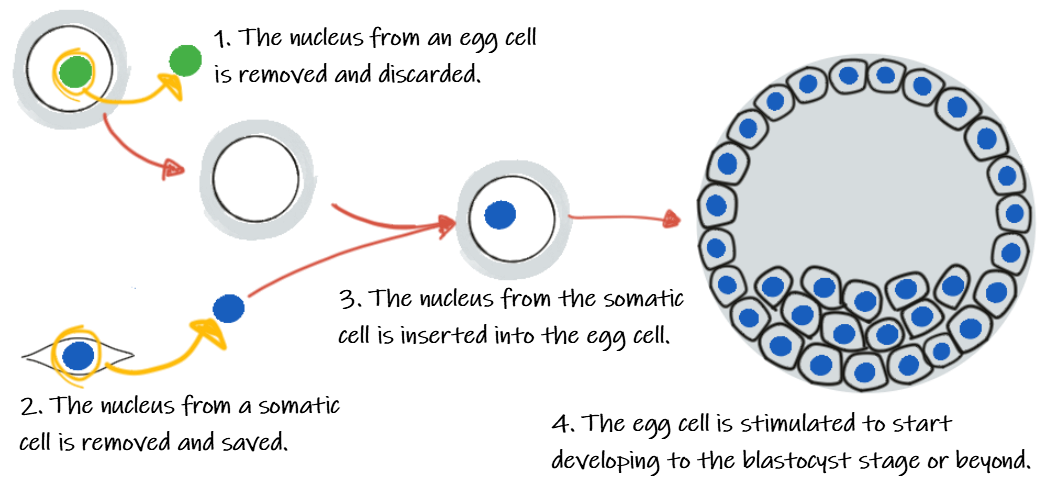
A major fear when Dolly was first created was that the DNA inserted into the recipient egg cell came from a six-year-old sheep, and therefore had the marks of “adult” DNA. Throughout life, DNA picks up epigenetic tags that influence gene expression, and telomeres, the protective repetitive DNA sequences that cap our chromosomes, get shorter over an organism’s life. In sperm and egg cells, the DNA gets reprogrammed to remove most such tags and lengthen telomeres, but a cloned organism’s DNA would never have gone through that process. It turns out that through largely unknown mechanisms most epigenetic tags are removed and telomeres are lengthened by the recipient egg cell. But the process in cloned organisms is inconsistent and sometimes incomplete compared to naturally derived embryos. While the large majority of cloned animals live normal healthy lives, differences in cloned organisms’ DNA has been documented and our understanding of all the effects of cloning on an animal’s health is still incomplete. This could also be why creating clones has been incredibly inefficient. A recent attempt to clone a macaque required 417 eggs and 63 surrogate mothers to produce just two viable offspring.
Future:
Make a new Fido, resurrect a species, or cure disease
Cloning has now been performed on at least a dozen different mammalian species. There are even companies that will produce a clone of your dog or cat for you. But before spending as much as $100,000 trying to recreate your beloved Fido or Whiskers, remember that development plays an incredibly important role in determining an organism’s physiology, behavior, and even appearance. Getting a cloned pet is less like getting your same dog back, and more like getting your dog’s identical twin. But for some other animals, cloning has started to go mainstream. Prize bulls used for breeding beef cattle can sometimes sell for hundreds of thousands of dollars. In these cases, a guaranteed identical genetic copy of a top sire may make the high price of cloning economically feasible.
Other scientists see cloning as a potential way to revive extinct species. For example, there has been a lot of talk around transferring the nucleus of woolly mammoths found frozen in the Siberian permafrost into the egg of the closely related Asian elephant. But DNA that has been frozen for thousands of years tends to not be in the best shape. While DNA from these cells has allowed us to sequence the mammoth genome, it is unlikely that DNA in the near perfect condition needed for cloning will be found. Still, for critically endangered species or for species that have gone extinct very recently, cloning could serve a conservation goal.
While human cloning has captured news headlines, as of 2018, creating a living, breathing, cloned human is still the stuff of science fiction, and is not believed to be the focus of any serious research. Research on so called therapeutic cloning with human cells is taking place, however. Therapeutic cloning aims at creating cloned human cells and growing them to the blastocyst stage. The blastocyst is then broken up and the cells are used for research, or perhaps one day, medical treatments. Researchers value these cells from early development, known as stem cells, because they have the potential to develop into any cell type in the body. Creating clones in this way could allow researchers to grow cultures of stem cells from a specific individual with a known genetic condition, greatly opening some avenues of research. Further in the future, such clones have the potential to be used in treating disease. Therapeutic cloning could one day allow physicians to create tissues from your own cells to use in your own treatment, virtually eliminating the risk of rejection by the body. But for reasons largely unknown, cells from primates, humans and their closest relatives, have proven especially difficult to clone. And because human egg donors must go through hormone treatments and surgery to obtain a relatively small supply of eggs, the amount of work that can be done on therapeutic cloning using human eggs is limited. But progress is being made, and for many scientists, the promise of genetically identical stem cells means that any obstacles that remain are worth working to overcome.
Questions
Review:
- Dolly was first mammal to be born that was an identical genetic copy of an adult organism. Some people just refer to Dolly as the first ever cloned organism. Is it wrong to do so?
- What makes mammals such difficult organisms to clone?
- Scientists feared that cloned organisms would be born “older” than organisms conceived naturally. Explain why this was a fear and whether that fear was borne out.
- Why may you expect a cloned pet to be different that your original pet in some ways?
- Why could creating cloned cells be helpful in potential medical treatments?
Critical thinking:
- While the large majority of cloned organisms have shown no health issues, some differences in their DNA have been identified. These differences stem from the incomplete removal of epigenetic tags and incomplete lengthening of telomeres in the donor DNA. Do you think that the offspring of clones, obtained through traditional breeding, would share these problems?
- A big problem in trying to save critically endangered species is trying to maintain genetic diversity –for example trying to avoid too much inbreeding to make sure that the remaining organisms aren’t too similar genetically. Would using cloning to help, in conservation add to or alleviate that problem?
Discussion:
- While studying therapeutic cloning has clear potential scientific benefits, some people oppose the practice because advances in therapeutic cloning techniques could also no doubt be used in reproductive human cloning, the actual birth of a cloned person. Do you think scientists should be responsible for how others use the techniques they have developed? Is it wrong for someone to study therapeutic cloning because someone else might use the knowledge they gain to do something unethical, like producing a human clone? How beneficial would the science have to be, or how bad would the potential unethical uses have to be to make you change your mind?
Answer key:
Available to teachers upon request: dnadots@minipcr.com
