Cancer immunotherapy
What it is:
Using the immune system to fight cancer
Doctors have had ways to kill cancerous tumors for a long time. They can remove cancers through surgery; they can kill them with radiation or chemotherapy. The weakness of these treatments is that it is very hard to recognize and target every last cancer cell among the trillions of other healthy cells of the body. This is a major reason why the side effects of cancer treatment are so notoriously awful—while trying to kill the cancerous cells, we also damage healthy parts of the body. It is also why cancer so often returns after treatment, as some cancerous cells are often missed.
But while it’s hard to target cancerous cells from the outside, researchers have begun to ask whether we can target them from within the body. The body is already equipped with exquisite mechanisms to identify foreign invaders—the immune system. Perhaps researchers could develop treatments that take advantage of the tools of the immune system to instead target the body’s own cells growing out of control, what we call cancer. The immune system is designed to quickly and efficiently recognize and destroy very specific cells and pathogens. Cancer immunotherapy is the group of treatments that directs the immune system to target cancerous cells. In some cases, the main goal is to recognize cancer and to specifically deliver therapeutic agents. Other immunotherapies go further, enlisting the body’s immune cells to fight the cancer directly. The use of these treatments has created some of the biggest breakthroughs in cancer treatment in decades. In 2018, the Nobel Prize in Physiology and Medicine was awarded to two researchers, James P. Allison, Ph.D and Dr. Tasuko Honjo, for their pioneering work in the field.
How it works:
Antibodies recognizing antigens
The immune system relies on the interaction of antigens and antibodies. An antigen is any molecule that the immune system recognizes as foreign, and it is usually a protein located on the outside of a cell or virus. Antibodies are proteins made by immune cells. Each type of antibody can identify a specific antigen, like a lock recognizes a key. Pathogens, such as a cold virus, make proteins that your body does not, and the immune system’s antibodies can recognize and target these proteins as foreign. By producing antibodies that can recognize these proteins as antigens, immune cells can target invaders for destruction. Antibodies can travel through the body on the surface of specific immune cells or be free floating on their own. When an antibody comes into contact with the specific antigen it recognizes, the two molecules bind together, and it is this binding that serves as a signal to the rest of the immune system to take action.
A major focus of immunotherapy is to take advantage of this very specific binding between antibodies and antigens. Once a researcher identifies a protein that is uniquely present on the outside of certain cancer cells, they can develop a specific antibody that will uniquely bind to this protein. These antibodies, called are made in a lab and then administered to a patient through injection or IV. The antibodies travel through the body and bind directly to the antigens when they encounter the cancerous cells. What happens next depends on the type of treatment. In some, these antibodies are designed to signal immune cells to come and kill the cancer cells. In others, the antibodies will carry a drug molecule that is released when the antibody binds its target antigen, targeting delivery of the drug directly to cancer cells and minimizing potential toxicity to other parts of the body. Yet in other types of treatments, antibodies are designed to just bind to the cancer and stay bound; if the antigen is a protein that influences tumor growth or recruits blood supply to a tissue, just binding to and blocking that protein can slow or stop cancer growth.
But our immune system also has the ability to recognize cancer cells using its own antibodies. The immune system’s ability to recognize and kill diseased cells is one reason we don’t get cancer more often. In some cases, however, cancer cells start to make other antigens, ones that signal to the immune system to hold its fire. These so-called immune system checkpoints are antigens that normally play a key role in switching off the immune response after infection, telling immune cells to stop attacking and ensuring that no immune response ever gets too big and out of control. Some cancer cells hijack these checkpoints, presenting them on their surfaces where they don’t belong and helping the diseased cells hide from the immune system. New treatments develop monoclonal antibodies that bind to the checkpoint molecule present on cancer cells. 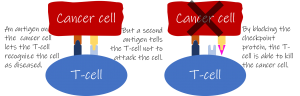 The checkpoint antigen now bound to the monoclonal antibody is blocked from signaling to immune cells to hold their fire. Immune cells will be free to attack the cancer without interference. By introducing such “checkpoint inhibitors”, immune cells can now recognize tumor cells and kill them.
The checkpoint antigen now bound to the monoclonal antibody is blocked from signaling to immune cells to hold their fire. Immune cells will be free to attack the cancer without interference. By introducing such “checkpoint inhibitors”, immune cells can now recognize tumor cells and kill them.
The future:
A cure for cancer?
The introduction of immunotherapy to the oncologist’s toolkit has been widely considered a revolution in cancer care. But most scientists agree we are still just at the opening stages of what is likely to be a much larger revolution. New treatments seek to go beyond adding antibodies as blockers or signals for the body and actually look to modify a patient’s own cells. CAR-T cell (chimeric antigen receptor T-cell) treatments, remove patients’ own T-cells and genetically modify them to produce antibodies that recognize specific cancer antigens. When these cells are reintroduced into the body and begin to bind to the cancer antigen, the cells are activated and not only can they kill the cancer, but they can also proliferate, creating even more cells dedicated to killing the tumor. The first CAR-T treatments were approved by the FDA in 2017, and there are likely more to follow.
But just like immunotherapy is a collection of related treatments, cancer is a collection of related diseases, and so different approaches have proved more or less effective depending on the specific disease and even the specific patient. And the best results are still typically obtained when immunotherapy is paired with more traditional treatments. Identifying which approaches work in different cases and understanding what differences between individuals make treatment more or less successful is a large focus of current research. For now, immunotherapy has been a gamechanger, but only for a few specific groups of cancer patients. Whether it can eventually become effective across all the diverse forms of the disease is something that scientists are working hard to uncover.
Questions
Review:
- What unique difficulties does cancer present that makes it different from other diseases?
- A typical cell has hundreds or even thousands of different molecules on its surface. What makes one of these molecules an “antigen”?
- What advantage would attaching a drug to an antibody have over administering the drug more generally?
- Immune system checkpoint inhibitors have been some of the most successful cancer immunotherapy treatments. Describe what the checkpoint is and what it means to inhibit the checkpoint.
- How are CAR T-cell treatments different from other antibody treatments?
Critical thinking:
- The use of checkpoint inhibitors tends to work best in cancers with higher numbers of mutations, such as melanoma, and less well with cancers that tend to have fewer mutations. With your understanding of the immune system and how antibodies recognize antigens, why may more mutations make a cancer an easier target for the immune system?
- One of the common side effects of immune therapy can be flu-like symptoms. Why may it make sense that stimulating the immune system makes you feel like you have the flu?
Discussion:
- Cancer immunotherapy has made a big splash in the media, giving hope to a lot of cancer patients. There are even significant amounts of prime-time advertising for immunotherapy treatments on television. But currently the number of diseases that these treatments have been approved to treat is small. Some doctors feel pressure from patients who face a difficult diagnosis to prescribe them these treatments even when they have not been approved for their specific disease. Unapproved treatments may very likely not work, and with any medicine there are possible side effects, some of which can be dangerous or painful. Furthermore, some medications can be extremely expensive – sometimes more than a half a million dollars, and if they have not been approved by the FDA, insurance likely will not cover the cost. But patients faced with a terminal diagnosis may be willing to try even unapproved treatments. Who should have the final say as to when patients get these unapproved treatments? Who should pay for the treatment if a patient receives it?
Answer key:
Available to teachers upon request: dnadots@minipcr.com
