Cell-free protein synthesis
What it is:
An easier method to make all the proteins we need
When someone has diabetes, their body is unable to produce the hormone insulin and can’t properly signal when to store nutrients. To make up for their lack of insulin, diabetes patients will instead inject themselves with insulin that has been manufactured in a lab. Insulin is a peptide hormone, which is a type of small protein. To make large quantities of this protein outside the body, scientists incorporate the DNA sequence for human insulin into host cells, such as bacteria or yeast, which then do the work of producing the insulin. This process of having host cells produce specific proteins in large quantities is an incredibly important technique for medical and research purposes. In the case of insulin, it has saved millions of patients.
Scientists have been making proteins this way for decades, and typically the procedure works well. For many reasons, however, sometimes host cells can be tricky to work with. Scientists have to constantly use expensive and complicated machinery to monitor the cells and make sure they are growing properly. The host cells are also still naturally producing their own proteins in order to stay alive. These other proteins can interfere with or delay the production of the target protein. Furthermore, once the protein has been made, it might be difficult to separate the target protein from all the other proteins and cellular components. In some cases, the target protein may itself be toxic to the host cell, making the host cells die before significant amounts of protein can be made. To help overcome all of these challenges, researchers have developed a method of expressing proteins that doesn’t require living cells, called cell-free protein expression.
How it works:
Cellular machinery without cells
Cells synthesize proteins according to the genes in the organism’s DNA through the processes of transcription —copying DNA into RNA—, and translation —reading the RNA to make proteins. Virtually all cells contain the biological machinery to do this. The enzyme RNA polymerase transcribes the DNA into mRNA. The ribosomes and tRNAs translate the mRNA to produce protein. Cells also contain the building blocks needed for the molecules they are making: nucleotides to build RNA and amino acids to build proteins. To power the reactions, cells produce the necessary ATP. Cell-free technology simply suggests that if you use all the right ingredients—the machinery, building blocks and energy source—you can perform transcription and translation without an actual cell. All a researcher has to do is add DNA with the gene they would like to transcribe and translate, then sit back and wait for their protein.
There are two major strategies currently used to make cell-free reactions. The first is to separately produce each cellular component that is needed and to then combine them all together into a single reaction. Some components, like nucleotides and amino acids, can be chemically synthesized. Other components, such as ribosomes and polymerases, still need to be produced by living cells and then separated from the cells. Since scientists have to individually create and purify each component, setting up this type of cell-free reaction is still complex and costly. However, because scientists are able to individually determine every molecule that is put into the reaction, they have tremendous control over the process which can result in high-quality proteins.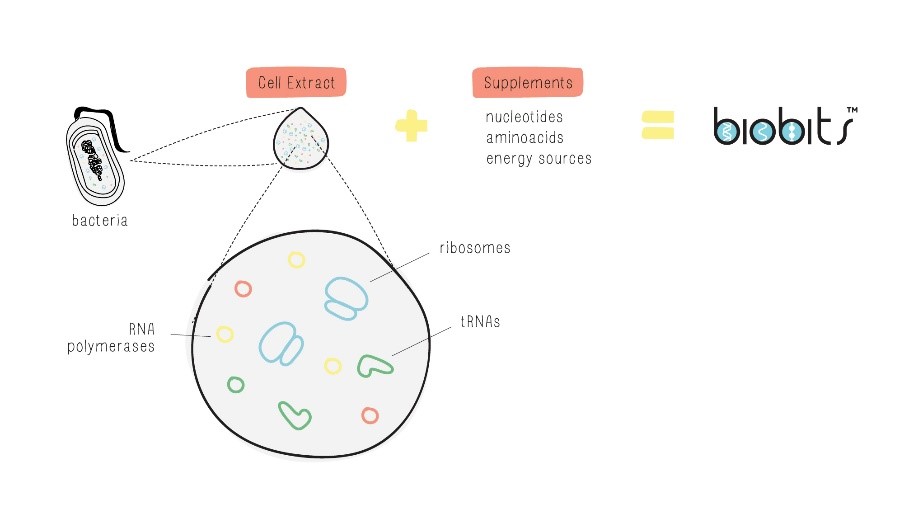
The second method is to extract all the components directly from host cells all at once. Scientists grow up a large amount of cells and then break them open through a process called lysis. In doing so, scientists can extract the polymerases, ribosomes, and other biological components needed for transcription and translation, and then supplement it with chemically-synthesized nucleotides, amino acids, and an energy source. This makes the entire process much simpler and more cost-effective, but it also results in a less purified reaction, as the extract will still contain many unneeded cellular components.
The future:
Expressing new proteins in new places
Since cell-free reactions don’t have cells membranes getting in the way, scientists can directly interact with and manipulate the different components in the reaction. This allows them to learn more and experiment with cellular processes that were previously too difficult to study in living cells. One example is to incorporate non-natural amino acids into the reaction. There are 20 naturally occurring amino acids, but scientists have been able to develop synthetic amino acids with unique chemical properties, and then use these non-natural amino acids to build new proteins in cell-free reactions that cannot be built in natural cells. In 2018, Kazutoyo Miura and their team used non-natural amino acids to develop a new malaria antigen, which is a small protein that mimics a pathogen used in vaccines to “train” immune systems to fight against specific diseases. The non-natural amino acids in this antigen allow it to bind strongly to immune cells, trigger an immune response, and train them to recognize similar pathogens in the future. With many parts of the world still suffering from malaria and other diseases, we need new vaccines and treatments; using non-natural amino acids may help us discover them.
Cell-free reactions don’t have cells that need to be kept alive, but they do contain sensitive molecules that require specific storage conditions. To get around this, scientists freeze-dry the reaction to make them last longer at room temperature. By freezing the reaction and then pulling all of the water out with a vacuum pump, they produce a dry solid that is stable outside of the freezer—similar to how beef left at room temperature will begin to rot, but beef jerky is stable for a long time. All the user has to do is rehydrate their reaction with water, add their DNA of interest, and transcription and translation will begin. Typically, pharmaceutical  companies will produce medically-relevant proteins in large batches and ship them on ice to the patients who need them. However, the live-cell production and cold shipping processes are expensive. Freeze-dried, cell-free reactions could be shipped instead so therapeutic proteins can be produced directly in small batches on-demand, virtually anywhere in the world, at a fraction of the cost.
companies will produce medically-relevant proteins in large batches and ship them on ice to the patients who need them. However, the live-cell production and cold shipping processes are expensive. Freeze-dried, cell-free reactions could be shipped instead so therapeutic proteins can be produced directly in small batches on-demand, virtually anywhere in the world, at a fraction of the cost.
Questions
Review:
- What basic steps have to take place for DNA to be made into protein? Name each step and give a one sentence explanation of the process that takes place.
- What difficulties do scientists face when trying to express a specific protein in living cells?
- Even though cell-free reactions do not contain cells, they do need to contain several essential components so proteins can still be expressed. List at least 5 biological components that a cell-free reaction needs (either extracted from living cells or supplemented by the scientists).
- What are 2 similarities between the two methods of creating cell-free reactions? What are two differences?
- What advantages does freeze-drying the cell-free reaction have over just the cell-free reaction alone?
Critical thinking:
- Membrane proteins are proteins that are embedded in the cell membrane, such as transport proteins that move molecules and ions in and out of the cell. What would be a potential challenge in expressing these proteins in live cells? What would be a potential challenge in expressing these proteins in a cell-free system?
- Live cells are able to constantly regenerate the necessary cellular components so they can keep producing proteins without running out of the resources they need. In a cell-free reaction, there are no live cells, so therefore, these reactions can run out of a necessary component. In order to make as much protein as long as possible, what component would you add the most of?
Discussion:
- The new vegan substitute for meat, the “Impossible Burger” contains a molecule called heme. Heme is found in the protein hemoglobin, which transports oxygen in the blood, gives blood its red color, and is part of what our brains interpret as the flavor of meat. Including heme allows the Impossible Burger to look and taste more like real meat. The heme used here is derived from soy plant DNA and is produced in large batches in yeast cells. Imagine instead that they used a DNA sequence from cows, but made the heme in a cell-free system. Would the burger still be vegetarian? Would you eat it if they used a human heme DNA sequence in a cell-free system?
Answer key: Available to teachers upon request: dnadots@minipcr.com
