Epigenetics
What it is:
Gene regulation that “learns from experience”
Often when you’re reading something that you know you’ll have to read again later, you highlight or cross out passages so the next time you can read it more efficiently. Your cells do something similar to DNA. Signals are put on DNA based on experiences you have, so the cell knows when it will or won’t have to express that gene later on.
Epigenetics means “above the gene”, and refers to changes to DNA that are not changes to the actual sequence of As, Ts, Cs and Gs that make up your genetic code. It is similar to how highlighting a sentence in a book may change how you view that sentence, even though you didn’t change anything about the actual order of words. Epigenetics is putting molecular signals on certain genes to switch them on or off. Epigenetic tags act as a kind of cellular memory.
All cells can turn up or turn down how much they use a particular gene, for example as cells take on specialized roles during development. The gene that codes for myosin, a main protein that makes up muscle fibers, is transcribed much more vigorously in skeletal muscle cells than in nerve cells. Likewise, different cell signaling cascades can turn genes on or off depending on environmental or other cues. This typically occurs when promoter regions in DNA interact with transcription factors, activators and repressors. Epigenetic modifications add an additional layer of transcriptional control in one key way: epigenetic changes make changes to gene expression long lasting, by definition being passed through at least one cell division. Usually, this means being passed through mitosis, where new cells in a particular tissue can inherit epigenetic tags made by their parent cells. Most such epigenetic changes are removed during gametogenesis (formation of sperm and egg), meaning that the offspring’s expression pattern begins as a clean slate. But some epigenetic tags have been reported to be passed through meiosis, from parent to offspring. In other cases, the parent can even put tags on the offspring’s genome that are not present in the parent. It’s as if when you highlighted a book, the next time it was printed your markings were already in it for the next reader.
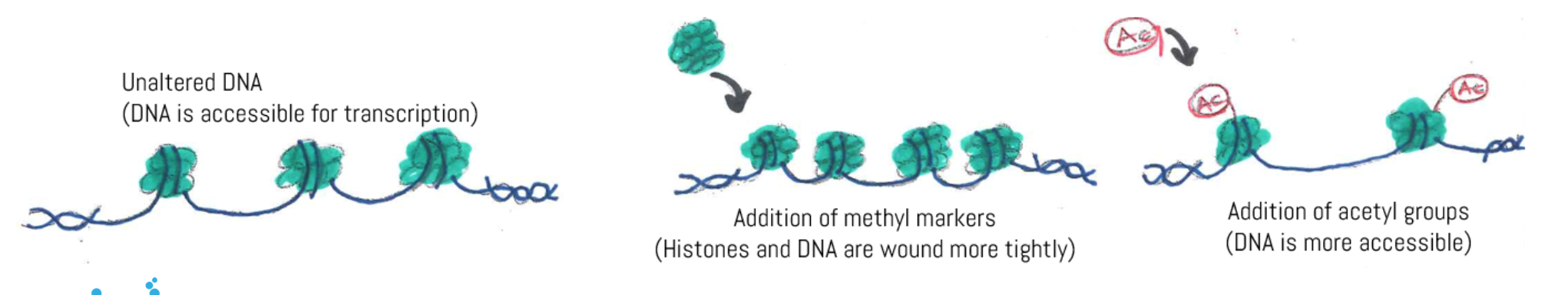
The two main forms of epigenetic tags are DNA methylation and histone modification. DNA methylation is the addition of methyl group (CH3) covalently bound to the DNA strand, usually to a cytosine. Methylated DNA is usually coiled tightly around histones, proteins used to wind and package DNA from a long linear double helix into a more compact structure, repressing gene expression. Histone modification involves adding acetyl groups (Ac) to histones, usually making DNA more accessible. The more tightly wound the DNA, the harder it is for genes to be expressed; the more loosely packed and stretched out the DNA, the more readily genes can be turned on.
A genetics mind shift:
Inheritable experiences?
The fact that epigenetic markers survive through cell division and, in some cases, are passed down to offspring throws a serious wrinkle in our understanding of how traits are inherited. Normally we think of phenotypic traits as being the direct result of our genotype, or the set of alleles that we received from our parents. But with the discovery of epigenetics, we now know that some inherited traits may actually be influenced by the environment. An excellent example is provided by children born during the Dutch Hunger Winter of 1944-1945. These children were born to mothers who experienced famine during early pregnancy. Multiple studies have found that these children, who are now adults, are at increased risk of coronary heart disease and obesity, despite living perfectly normal lives themselves.
This suggests that a mother’s life experiences, in this case a severe nutritional deficiency, can be passed down to her child, forever altering the child’s phenotype.
This is not to say, however, that all experience is passed on. The number of traits known to display epigenetic inheritance in humans is still relatively small. It also is not to say that an individual’s genotype is not important. Traits under epigenetic control may show a range of phenotypes for any one genotype, but that range is limited, and the limits are still very much under genetic control. Still, our understanding of epigenetics has helped explain some patterns of inheritance that had long puzzled geneticists.
The future:
New epigenetic-based medical treatments
Changing epigenetic expression patterns has promise for treating several previously difficult to treat diseases and disorders. For example, research is being done to more easily treat PTSD and other anxiety disorders. Regular treatment for these disorders often involves exposure therapy, confronting the object that causes fear or anxiety in a safe environment. Exposure therapy has previously been associated with epigenetic modification of two genes. Drugs are currently being developed to possibly make exposure therapy more effective by decreasing expression of those genes through epigenetics. Epigenetic therapies are also being developed for cancer. Research suggests epigenetic mechanisms are important in how cancers silence antitumor systems and activate cells for rapid growth. The FDA has already approved two drugs to treat cancer by reactivating these antitumor systems. The list of epigenetic treatments for cancer, neuropsychiatric disorders, and other ailments is currently small, but it is growing. How large the field of epigenetic medicine will become is still an open question.
Learn more:
- “Epigenetics.” Wikipedia. https://en.wikipedia.org/wiki/Epigenetics
- Weinhold, Bob. “Epigenetics: The Science of Change.” Environmental Health Perspectives. http://www.ncbi.nlm.nih.gov/pubmed/16507447
Questions
Review:
- If the prefix “epi” means “on top of”, in what ways is epigenetics “on top of” regular genetics?
- Describe the two basic mechanisms for how epigenetic changes are made.
- In what way do epigenetic changes modify the gene expression patterns driven by transcription factors?
- Are all epigenetic changes inherited from parent to offspring?
- If anxiety and conditions like PTSD are at least partially controlled by epigenetic factors, why may traditional
drugs that treat the symptoms of these disorders be less effective or long lasting than changing methylation
patterns in the person’s DNA?
Critical thinking:
- Propose a simple epigenetic model for why children born to mothers suffering from low nutrition may be more
prone to heart disease and obesity than children both to well-nourished mothers. - Epigenetics is sometimes argued to go against Darwin’s theory of Evolution by Natural Selection. Some
suggest that epigenetics even points towards Lamarck’s concept of change by inherited acquired
characteristics. Explain why this is not the case.
Discussion:
- We now know that nutrition, chronic stress, and even some environmental chemicals have the potential to
change our physiology through epigenetics with effects that could possibly last for generations. We also
know that exposure to such risk factors is highly correlated to poverty. As a society, what obligations do you
think we have to combat such risk factors? Does knowing that poverty in one generation could potentially
affect the health of multiple generations to follow affect how you answer this question?
Answer key:
- Available to teachers upon request: dnadots@minipcr.com
