Gene Drive
What it is:
Cheating genetic probability
Normally, when you flip a coin there is a 50:50 chance of getting heads or tails. But there are ways to cheat. You could use a biased coin: one that is weighted, or even a coin where both sides are heads. When an organism reproduces sexually, it passes on only half of its DNA; like flipping a coin, which half is the lucky half to be passed on is left to chance. This concept is a founding principle of Mendelian genetics. Gene drives cheat this rule of ordinary genetics, acting like a biased coin, promoting the inheritance of a particular gene to increase its prevalence in a population. Gene drive mechanisms were first noticed occurring naturally in mice, flies, beetles, and other organisms. In these examples, specific alleles increase their own transmission selfishly and at the expense of alleles located on the homologous chromosome. It’s as if the coin is weighted, so that one side will land up more often. Scientists have now realized that they can utilize this naturally occurring phenomenon to their own advantage. As each generation reproduces, scientists can create a scenario where only one of the two alleles for a given gene is passed on – they can choose which side of the coin lands facing up. In fast reproducing species, such as many insects, these engineered alleles could drive their own spread through populations at tremendous speed, outnumbering naturally occurring alleles within generations.
How it works:
Cut and repair DNA
Imagine that there are two flies; one fly has a gene drive in one of their chromosomes and the other has normal chromosomes. They mate and produce an offspring which has one chromosome with a gene drive and one normal chromosome. The gene drive system cuts the normal chromosome. To repair the broken chromosome, the cell uses the intact chromosome, the one that holds the gene drive, as a template. In doing so, the newly repaired chromosome will now also contain the gene drive. Now that both chromosomes contais a gene drive, the fly is guaranteed to pass it on to its own offspring. This pattern will continue, spreading through the population until only gene drive chromosomes are left. It’s as if the heads side of the coin was able to copy itself, replacing the tails side.
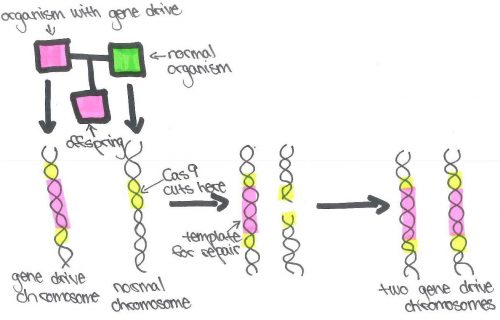
The most common type of gene drive systems cut DNA with endonucleases, enzymes that break the covalent bonds between nucleotides in a DNA strand as described above. Cas9 and Cpf1, endonucleases associated with CRISPR, are often used. In this scenario, a chromosome is created containing three basic parts: Cas9 or Cpf1, a sequence that creates an RNA that will recognize the target sequence on the other chromosome, and finally a genetic payload, the sequence the scientist is interested in introducing into the population. When the drive chromosome is paired with a wild chromosome, the RNA will recognize the sequence on the opposite chromosome; the endonuclease will cut the chromosome where the RNA binds; and finally, the chromosome will be repaired using the drive chromosome as a template, introducing all three parts, including the genetic payload onto the newly repaired chromosome.
The future:
Reduce the spread of disease, eliminate invasive species
Imagine introducing a gene in mosquito populations that made their carriers unable to spread malaria. Such an innovation could literally save over a million people every year, mostly children under the age of 5 who are particularly vulnerable to malaria infections. Gene drive technologies hold this type of promise. They also could eradicate invasive species. There are proposals in New Zealand to introduce gene drive mechanisms to invasive mammals that would make females sterile. As these drive alleles spread, in every generation there would be fewer and fewer unaffected females left to mate, driving the organism to extinction. In a place like New Zealand, where rare native populations are extremely threatened by invasive mice, rats, and possums, gene drives could do the work eradicating these species that humans have been unable to do on their own. In these ways, gene drive mechanisms hold promise to tackle complex ecological and epidemiological problems that are beyond the scope of traditional methods.
But there are risks. There are concerns that gene drives could escape the target population in which they are introduced. While hybridization between species is rare, aggressive genetic elements such as gene drives could cross species lines even through limited contact. And while eliminating pests or disease-causing organisms may sound appealing, the elimination or permanent altering of a wild species is something that can’t be undone, and there will no doubt be unintended effects of doing so. Mathematical modeling and computer simulations can help mitigate much of this risk, but never all of it. There are also concerns that gene drives won’t be as successful in natural populations as they are in the lab. Populations that don’t display Mendelian inheritance will be under tremendous selective pressure to evolve modifiers, alleles that can overcome the effect of the drive. While the drive spreads through the population, so will organisms resistant to drive. Genetic drive mechanisms hold tremendous potential to address complex ecological and epidemiological problems, but whether they will be successful, or if they should be used at all, is still an open question.
Questions
Review:
- Why is a gene drive system similar to a trick coin?
- How does cutting a chromosome in two lead to a change in the DNA sequence?
- What is a “genetic payload”? What could be some examples scientists may be interested including as a genetic payload?
- Why are mosquitoes considered a worthy target for genetic drive systems?
- Why is it likely that strong selective pressures may stop the spread of genetic drive mechanisms?
Critical thinking:
- Why would a gene drive mechanism evolve naturally? Who or what benefits in a gene drive system, the gene drive mechanism, the individual organism, the population?
- If a scientist introduced a gene drive mechanism that made females sterile, how would it spread through the population? Wouldn’t the fertile females be the organisms having all the offspring?
Discussion:
-
Should there be limits put on how gene drives are used? If so, what should they be? Are some uses worth the risks? Is permanently genetically altering wild populations beyond what humans should be allowed to do?
Answer key:
- Available to teachers upon request: dnadots@minipcr.com
